Floral litmus solutions
The week of chemistry began with a review of atomic structure which led into a highly abbreviated introduction to bonding and molecules.
Monday began with an introduction to the periodic table and a fast slide into electron orbitals.
Wednesday then focused on orbital filling and the connection to bonding, to chemistry.
This term I reversed the table order so that the whiteboard, read from left to right, matched the table activity. This worked well.
The floral bleach turned out to be buffered in some way such that the bleach was not basic. That was a fail and will need to be replaced.
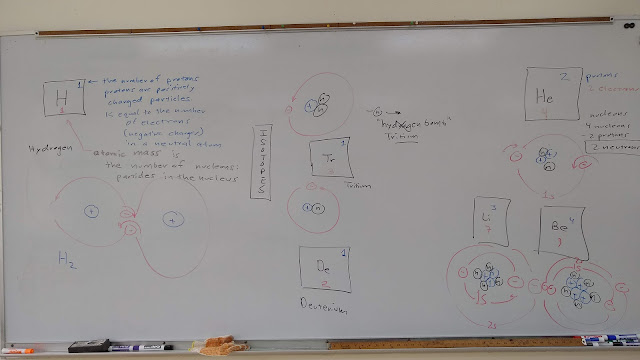
Board notes
The 11:00 section arrived sans flores. Most of the students used leftover floral pigment solutions. Boiling Sphagneticola trilobata did not yield acid detection capability. Acid only decreases the saturation, not a reliable indicator. The flower does shift yellow to orange in the presence of a base.
Monday began with an introduction to the periodic table and a fast slide into electron orbitals.
Wednesday then focused on orbital filling and the connection to bonding, to chemistry.
Jerome works out whether his flower can detect both an acid and a base
This term I reversed the table order so that the whiteboard, read from left to right, matched the table activity. This worked well.
Dori-Ann makes notes on color changes for her flower.
The floral bleach turned out to be buffered in some way such that the bleach was not basic. That was a fail and will need to be replaced.
Pine Cleen [sic] was a new addition along with the clear Joy detergent (which is neutral). The cream of tartar ran out.
Merenda preps her flower
Franky and Merenda working on identifying unknowns
Board notes
The 11:00 section arrived sans flores. Most of the students used leftover floral pigment solutions. Boiling Sphagneticola trilobata did not yield acid detection capability. Acid only decreases the saturation, not a reliable indicator. The flower does shift yellow to orange in the presence of a base.
Shawn and Ann-Julie
Bredalyn, Delinah, and Rosalyn work out color changes
LcRose, Emelisse, Janice Stacia, and Rosalyn (Bobo, Jan, Dass, Sinotwe)
LcRose

















Comments
Post a Comment