Acids, bases, atomics
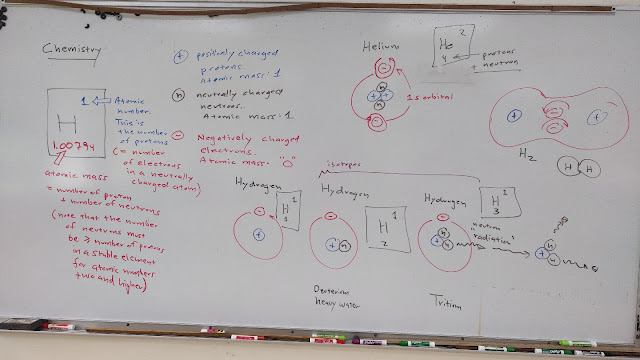
Monday of the week on chemistry began with an introduction to Hydrogen.
After an introduction to isotopes, the lecture moved all too quickly into orbitals and lithium. As I had noted last fall, by the 40 minute mark the students were at the limit of their ability to pay attention, information saturation had been hit. Last spring a tropical system was developing in the waters of Pohnpei. I shifted gears and covered the developing weather system. By the next morning, Thursday, classes had been canceled and part of Kitti were cut off by downed trees.
Thursday April 20, 2023
Trees were also down on the road over the pass into Palikir.
This pushed the laboratory back a week in spring and dropped the second day of atomics lecture.
Wednesday I backed up a little and tackled beryllium followed by sodium. I wanted to use sodium and chorine to get to NaCl, salt. The problem is Chlorine is complex. I tried to run to the concept of a Neon core, but that was perhaps a bridge too far for the students.
I then turned back to methane, carbon dioxide, and water. I used the ptable displaying on the smartboard. I did manage to end with hydroxide and hydrogen ions.
Thursday's lab opened with the brief review of on the far left side of the atomic underpinnings for acids and bases. Again this term I brought in sufficient Spathoglottis plicata to provide for the class. Only a couple students brought flowers.
The right board at class start.
Again this term I used the dimethyl benzyl ammonium chloride as the known base. This is stronger and more consistent than baking soda. The baking soda this term, being old, tested as neutral for all floral litmus solutions today. Baking soda seems to be a very weak base at best and prone to loss of potency.
Aryolynn and Darian identifying unknowns
Ruth records data as Aryolynn tests an unknown
Darian detects an acid while Ruth records results
Darian preparing test tubes
Alyssa, Ivy and Leriangelica test unknowns
Stella and Memichin work in the morning on unknowns
Pevirleen tests baking soda
Memichin runs tests while Mia records data
An 11:00 student brought in coleus, koromahd, which started off rosy brown with an acid detection being magenta and a base being lawn green. After a bit more boiling the starting color shifted to a darker reddish coloration near the X11 color Indian Red.























Comments
Post a Comment