Elements and floral litmus solutions
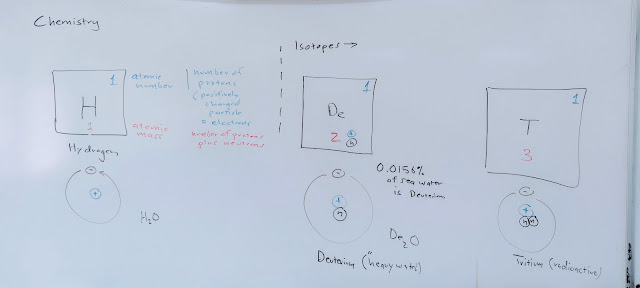
Monday began with an introduction to the periodic table and the pseudo-structure of atoms.
Then I moved on to higher elements and orbitals. No, this is not the quantum reality, but a beginners mind is not ready for a quantum approach.
Wednesday I moved on to bonding, starting with hydrogen-hydrogen bonding to fill the 1s shell. No, the orbitals are not correct, but they provide a visual model on which to hang the ideas of the valence shell structure. The leftmost NaOH was the end of my notes, the H₂ bonding was erased.
These diagrams all have their issues...
Mercedes testing a floral solution for a color change due to the addition of a known acid
Thursday the students worked with using floral litmus solutions to identify whether substances were an acid, base, or neutral.
Derisalynn found that her flower had insufficient pigment to be used
When a student has a flower that does not respond to the known acid and bases
The known acid was lime fruit
Insufficient pigment to detect color changes
The colorless solution was Heterotis rotundifolia and Ixora coccinea.
At 8:00 I put up the X11 colors. This proved problematic because Hibiscus rosa-sinensis matches rosy brown over around 30 degrees, which lead to misinterpretation of the results. Mercedes and Josephine are trying to match a color. At 11:00 I used a color wheel image from WikiMedia that did not lead to this confusion.
I had expected this bathroom cleaner to be basic, but it is an acidic cleaner based on citric acid.
Mercedes and Marcia working on identifying unknowns
Marcia with a positive test for an acid
Herna taking notes on results from Mikie
Destiny Grace identifying unknowns
Faithlyn takes notes, WikiMedia image seen in the background
Johannes
Melrose working with Keiko-Lalii
Unknowns
A new bottle of vinegar this term
Replenished bottle of rubbing alcohol
New this term: chalk, Lysol, and shampoo
The chalk proved to be neutral. The Lysol, as noted above, was a surprise when it proved to be acidic. Household cleaners have been basic. Soap, detergent, PineSol, ammonia, are all basic. Because soaps are basic, I had expected the shampoo to be basic, but this particular shampoo was rather strongly acidic. The hydrogen peroxide is out, but that always came up neutral. As a weak acid and in a very dilute form, H₂O₂ is too weak to cause a color change in the floral litmus solutions and tests as being neutral.
8:00 board diagram
Second board

























Comments
Post a Comment